Angelman Syndrome
UNC Brain Development Study of Infants with Angelman Syndrome
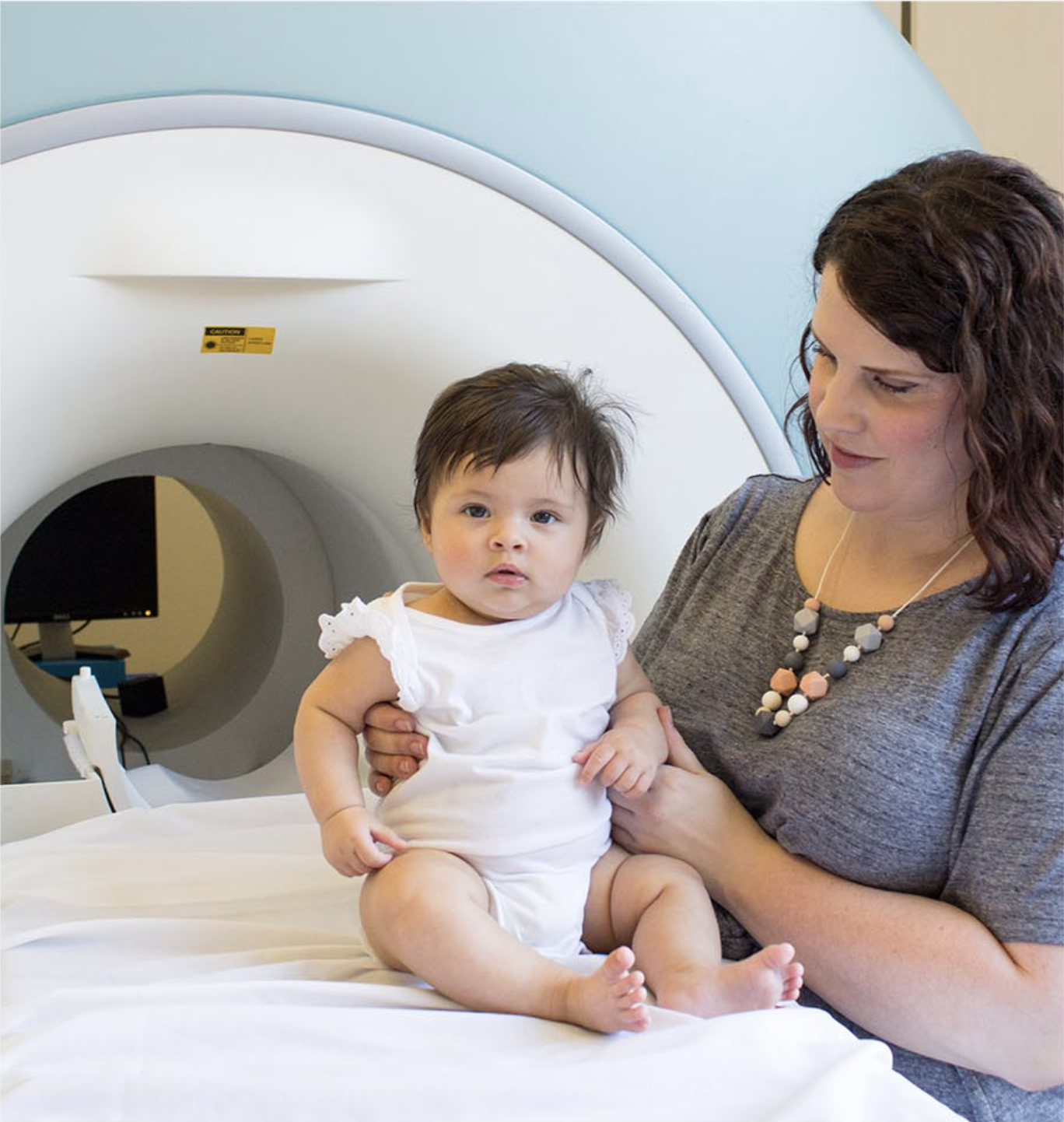
The Shen lab is recruiting for a study of brain development in babies with Angelman syndrome (AS). We are trying to identify the earliest brain and behavioral features of AS, and you can help us by participating in the first MRI research study of its kind. These babies will be compared to infants with fragile X syndrome (FXS), infants at higher likelihood for autism, infants with typical development, and infants with Down syndrome.
Goals of this research:
The goal of this research is to understand the earliest detectable neurological biomarkers to improve early detection and inform potential treatments for neurodevelopmental disabilities.
Who is eligible?
Infants with AS who are under 24 months of age.
What does participation involve?
Traveling to UNC for two in-person visits: (1) the first visit between 6-12 months of age; (2) if possible, a second visit at 24 months of age.
Behavioral and cognitive assessments at each visit (at no cost to the family) - with a summary of results for the family.
A safe, non-invasive MRI brain scan during natural sleep (no anesthesia or sedation involved) - with a copy of the MRI for the family.
You will receive:
All travel costs to Chapel Hill, North Carolina will be covered by the study along with compensation of up to $200.
Contact to participate:
If you are interested in enrolling or have any questions, please fill out the interest form OR reach out to our study coordinator:
Email: natalie_claypool@med.unc.edu
Phone: INSERT
Insert flyer above^
Clinical Trials for Angelman Syndrome
The CIDD Clinical Trial Program (co-directed by Mark Shen) is conducting two Phase 1/2A clinical trials aimed at evaluating the safety and tolerability of genetic treatments to restore the function of UBE3A.
Goals of this research:
INSERT
Who is eligible?
INSERT
What does participation involve?
INSERT
You will receive:
INSERT
Contact to participate:
If you are interested in enrolling or have any questions, please fill out the interest form OR reach out to our study coordinator:
INSERT